Our Technology
At Genesis TMS, we use FDA-approved, non-invasive Transcranial Magnetic Stimulation (TMS), enhanced by the breakthrough intelligence of FirstPath AI. This means your treatment isn’t just cutting-edge—it’s designed around you.
FirstPath AI helps us deliver more precise and effective care for depression, OCD, and other approved conditions, giving patients new hope when other treatments haven’t worked. Even better, this advanced therapy is now covered by most major insurance plans, so life-changing care is within reach.
The Science of Modern TMS
Neuroimaging and electrophysiological studies demonstrate that TMS can normalize dysregulated activity in circuits governing mood regulation, executive control, and emotional processing. By inducing long-term potentiation and depression at the synaptic level, TMS facilitates durable neuroplastic changes that underlie clinical improvement.
This convergence of neuroscience, technology, and clinical research positions TMS as one of the most advanced, evidence-based neuromodulation therapies available in modern psychiatry.
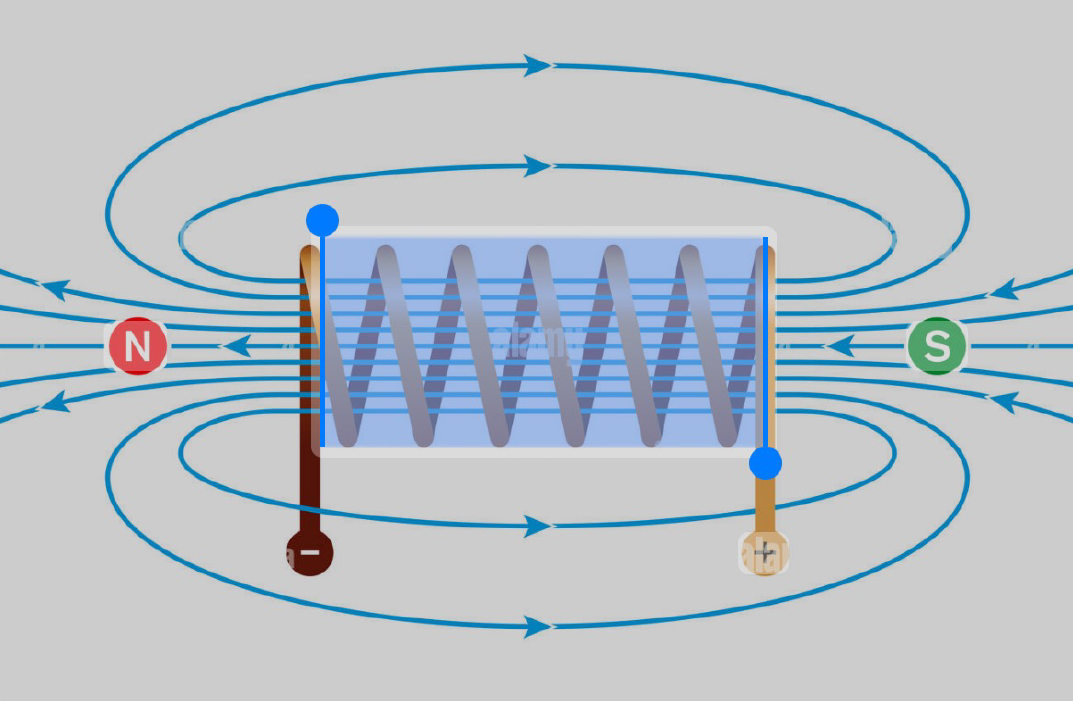
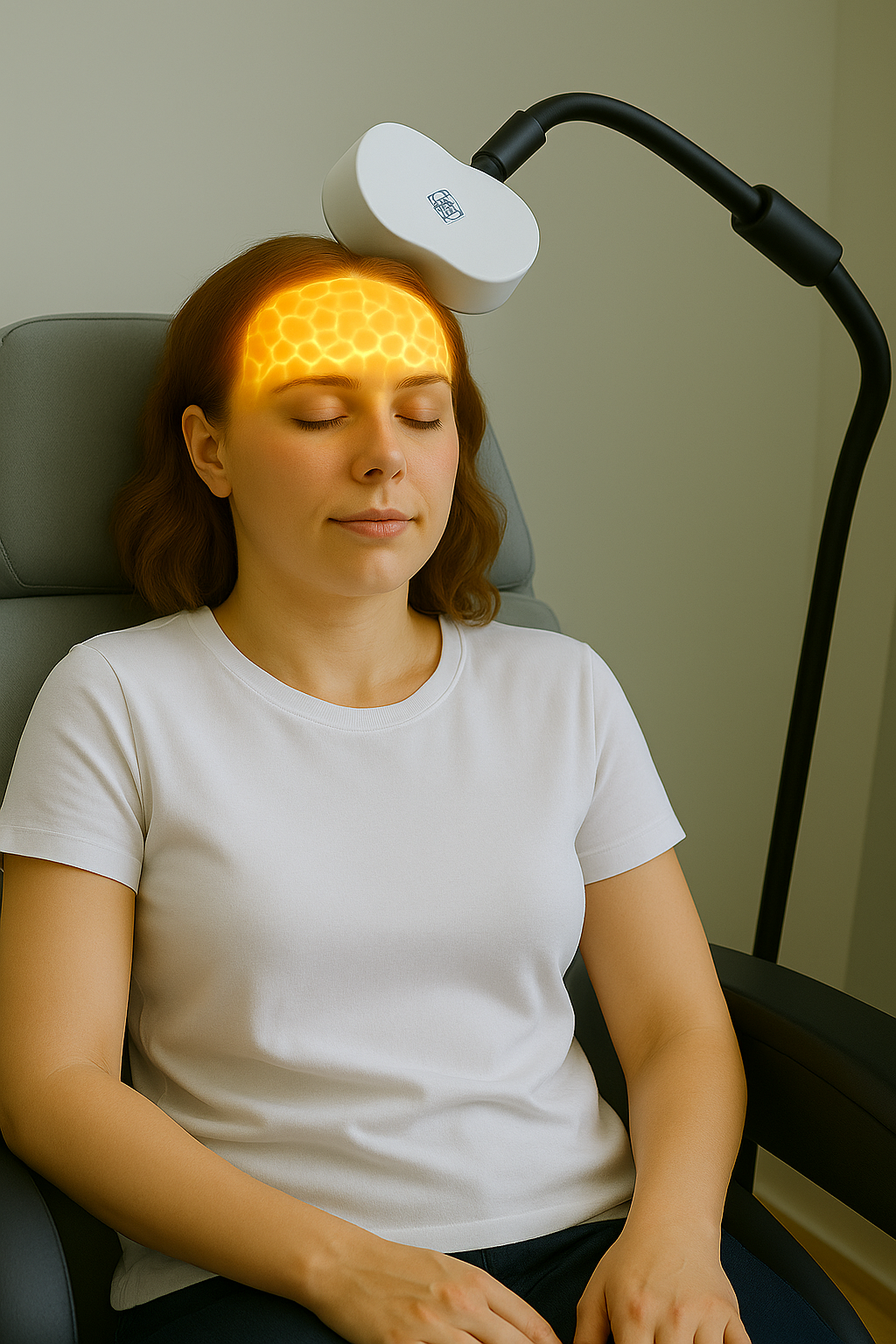
The Treatment Experience
 Pre-Treatment Brain Mapping Each patient undergoes precise brain mapping, incorporating advanced imaging and neuro-navigation to identify individualized stimulation targets.
Pre-Treatment Brain Mapping Each patient undergoes precise brain mapping, incorporating advanced imaging and neuro-navigation to identify individualized stimulation targets.
 Personalized Protocol Development Treatment parameters are tailored to your unique neurophysiology, ensuring optimal outcomes while minimizing unnecessary stimulation.
Personalized Protocol Development Treatment parameters are tailored to your unique neurophysiology, ensuring optimal outcomes while minimizing unnecessary stimulation.
 Comfort-Focused Design Our systems are engineered for patient comfort, with adaptive protocols and continuous evaluation to ensure a positive treatment experience.
Comfort-Focused Design Our systems are engineered for patient comfort, with adaptive protocols and continuous evaluation to ensure a positive treatment experience.
 Real-Time Response Monitoring We track neural and clinical response patterns in real time, allowing ongoing refinement of treatment to maximize effectiveness.
Real-Time Response Monitoring We track neural and clinical response patterns in real time, allowing ongoing refinement of treatment to maximize effectiveness.
 Mobile Companion App Patients gain access to an integrated app that supports progress tracking, appointment management, and evidence-based mental health resources.
Mobile Companion App Patients gain access to an integrated app that supports progress tracking, appointment management, and evidence-based mental health resources.
Our Advanced TMS Systems
Genesis TMS leverages next-generation neuromodulation platforms, representing the forefront of TMS technology in 2025. These systems integrate AI-driven precision targeting, adaptive stimulation algorithms, and real-time data analytics to deliver unparalleled accuracy, personalization, and clinical outcomes.
Accelerated Protocols
We offer Stanford-developed accelerated TMS protocols that can significantly reduce treatment duration while maintaining or improving efficacy:
- Standard protocol:
36 sessions over 6-8 weeks. - Accelerated protocol: 10 sessions over 5 days, followed by maintenance sessions.
Expanded Treatment Applications
- Major Depressive Disorder (MDD) – Our primary FDA-approved application.
- Treatment-Resistant Depression –For patients who haven’t responded to medications.
- OCD –
FDA-approved for obsessive-compulsive disorder. - Smoking Cessation – Helping reduce cravings and dependency.
- Post-Traumatic Stress Disorder – Alleviating symptoms through targeted stimulation.
- ADHD

